Galectins
Galectins constitute a family of multifunctional galactoside-binding lectins. They are widely expressed in various types of human cells and participate in many cellular functions, including cell differentiation, cell–cell interactions, cell growth, adhesion and immune response. Likewise they are also known to contribute to different key events in tumorigenesis and tumor progression, such as differentiation, angiogenesis, malignant transformation, apoptosis, tumor immune escape and resistance to anticancer drugs. Accordingly expression of galectins in human colon shows significant changes during colorectal cancer development and metastasis. Therefore, selective targeting of the potentially harmful effects of galectins, as well as enhancing the action of tumor suppressing members of the galectin family represents an attractive option for the development of new treatments for colorectal cancer.
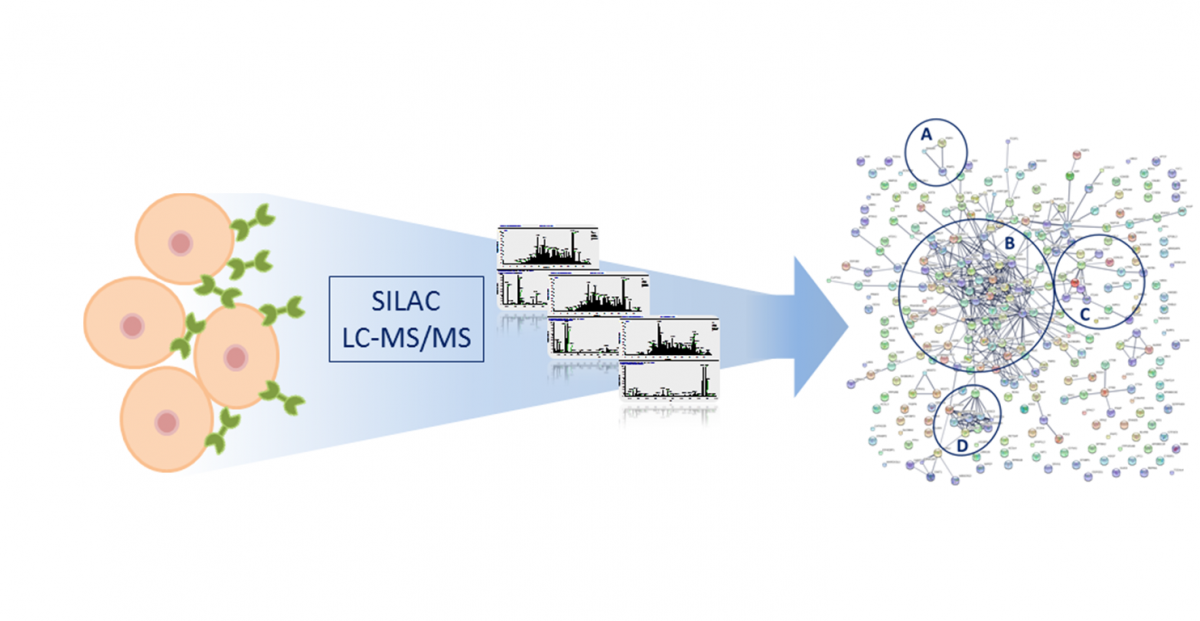
Current projects
Publications / Output
Katzenmaier EM, Stark HJ, Gebert J, Kopitz J. Galectin-12 colocalizes with splicing factor-rich speckles and shuttles between the nucleus and cytoplasm in colon cancer cells. Journal of Molecular Biochemistry (2018) 7, (28-40).
Katzenmaier EM, Kloor M, Gabius HJ, Gebert J, Kopitz J. Analyzing epigenetic control of galectin expression indicates silencing of galectin-12 by promoter methylation in colorectal cancer. IUBMB Life. 2017 Dec;69(12):962-970
Ludwig AK, Michalak M, Shilova N, André S, Kaltner H, Bovin NV, Kopitz J, Gabius HJ. Studying the Structural Significance of Galectin Design by Playing a Modular Puzzle: Homodimer Generation from Human Tandem-Repeat-Type (Heterodimeric) Galectin-8 by Domain Shuffling. Molecules. 2017 Sep 19;22(9). PMID: 28925965
Michalak M, Warnken U, André S, Schnölzer M, Gabius HJ, Kopitz J. Detection of Proteome Changes in Human Colon Cancer Induced by Cell Surface Binding of Growth-Inhibitory Human Galectin-4 Using Quantitative SILAC-Based Proteomics. J Proteome Res. 2016 Dec 2;15(12):4412-4422.
Lee J, Katzenmaier EM, Kopitz J, Gebert J. Reconstitution of TGFBR2 in HCT116 colorectal cancer cells causes increased LFNG expression and enhanced N-acetyl-d-glucosamine incorporation into Notch1. Cell Signal. 2016 Aug;28(8):1105-13. Epub 2016 May 5. PMID: 27156840
Katzenmaier EM, André S, Kopitz J, Gabius HJ. Impact of sodium butyrate on the network of adhesion/growth-regulatory galectins in human colon cancer in vitro. Anticancer Res. 2014 Oct;34(10):5429-38.
Kopitz J, Fik Z, André S, Smetana K Jr, Gabius HJ. Single-site mutational engineering and following monoPEGylation of the human lectin galectin-2: effects on ligand binding, functional aspects, and clearance from serum. Mol Pharm. 2013 May 6;10(5):2054-61. PMID: 23581621
Kopitz J, Ballikaya S, André S, Gabius HJ. Ganglioside GM1/galectin-dependent growth regulation in human neuroblastoma cells: special properties of bivalent galectin-4 and significance of linker length for ligand selection. Neurochem Res. 2012 Jun;37(6):1267-76. doi: 10.1007/s11064-011-0693-x. Epub 2012 Jan 11. PMID: 22234579
André S, Kaltner H, Lensch M, Russwurm R, Siebert HC, Fallsehr C, Tajkhorshid E, Heck AJ, von Knebel Doeberitz M, Gabius HJ, Kopitz J. Determination of structural and functional overlap/divergence of five proto-type galectins by analysis of the growth-regulatory interaction with ganglioside GM1 in silico and in vitro on human neuroblastoma cells. Int J Cancer. 2005 Mar 10;114(1):46-57. PMID: 15523676
Kopitz J, André S, von Reitzenstein C, Versluis K, Kaltner H, Pieters RJ, Wasano K, Kuwabara I, Liu FT, Cantz M, Heck AJ, Gabius HJ. Homodimeric galectin-7 (p53-induced gene 1) is a negative growth regulator for human neuroblastoma cells. Oncogene. 2003 Sep 18;22(40):6277-88. PMID: 13679866
Kopitz J, von Reitzenstein C, André S, Kaltner H, Uhl J, Ehemann V, Cantz M, Gabius HJ. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem. 2001 Sep 21;276(38):35917-23. Epub 2001 Jul 12.PMID: 11451961
Kopitz J, von Reitzenstein C, Burchert M, Cantz M, Gabius HJ. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J Biol Chem. 1998 May 1;273(18):11205-11. PMID: 9556610




